It was reported that Taytulla, a birth-control treatment recalled by Allergan due to incorrect packaging. Taytulla makers have announced the nationwide recall in the United States about the incorrect pills in the packets which can cause unintended pregnancies. In a statement issued by Allergan, it was stated that four pills were packaged wrongly.
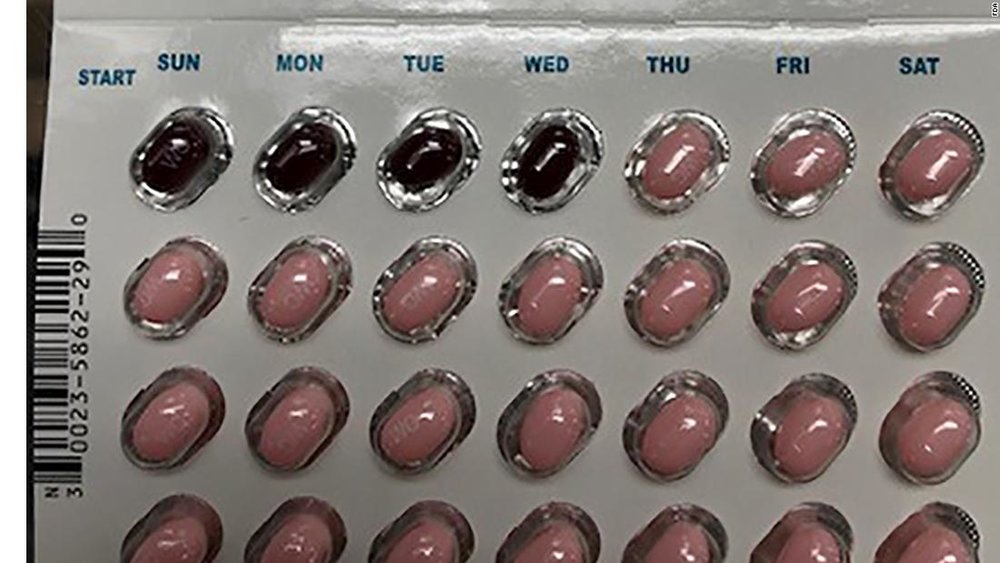
It was further reported that the packaging should have 24 pink capsules followed by hormones and then followed by four maroon capsules without hormones. But in the wrong packaged pills, four maroon pills were placed instead of 24 pink pills, as stated by Allergan. Improper placement of oral contraceptive capsules may put the people at risk of unintended pregnancies and contraceptive failure.
According to Allergan, the company is taking back all the wrongly packaged pills having lot number 5620706. The pills had an expiry date of 19 May. The company urged the parents to consult the physicians if they had used the capsules. On Tuesday, it was stated by Taytulla maker, Allergan that there were total 170,000 packs and were wrongly packed by a physician.
The company feared that the user may not notice the reverse order of the pills and therefore can face difficulties like unintended pregnancies. These packages are in the market since August. However, the drug-maker is voluntarily recalling the units.
Allergan is notifying the patients by writing letters to them and ask them to consult their physician. It was further stated that the shares of the company dropped 2% after the company recalled the packages. There are 28 total capsules in the packet from which 24 are active pink soft gel capsules which are used for the first 24 days and ‘WC’ is printed on the outer shell of the capsule.
While the other 4 pills are maroon soft gel capsules which are used for the next 4 days and ‘WC’ is also printed on the outer shell of the capsule. It was stated in the press release of Allergan that if any US patient used the pills having package number 5620706 and the 19 May as expiry date, then the patient should immediately consult the physician.
Anyone having 5620706 as the lot number should reach to their physician for rearranging the sequence of the pills. It was further stated by the Allergan that if anyone wants to know about these pills, he or she can contact at 800-678-1605 from Monday to Friday. The effects posed by the use of these wrongly sequenced pills can be noted to FDA’s MedWatch Adverse Event Reporting Program via e-mail.
The hormones which are present in the first 24 pills are necessary for the development of pregnancy and the rest four are required for further development. When a patient takes hormones which are required after 24 days, it disturbs the normal pregnancy process and patients may face an unintended pregnancy. It was asked from the Allergan about how many pills were recalled from the market, but the company officials did not comment on it.
The company has bad publicity as it was buffeted for months due to its dealing with a Native American Tribe. The company also cuts down 14,00 jobs in January when the company was prepared to face the generic competition from Restasis eye treatment.
When a woman is pregnant, the expectant mother always makes sure that they live as healthy as they can for their unborn child. They would often be prescribed by all the necessary vitamins and other drugs by their obstetrician in order to ensure that the unborn child will stay healthy and would continue to grow and be ready when they come out of the real world.
Therefore it is necessary for the pregnant women to not take the pills which may lead to future problems for them. As mentioned by the company, these pills are packed in reverse sequence and if a user is new, he/she may not notice the sequence of the pills.
Due to misplace order of the hormones, patients can endure unintended pregnancies, as reported by the company officials. There are specific hormones which are required on specific days, therefore the sequence of the pills is very important. To avoid any mishap, consult the physician if anyone has used these pills.




